|
The study of chemistry requires students to apply a vast amount of content knowledge to higher order thinking questions using a set of answering techniques. How can we score in Chemistry then? 1. Know the Essential Facts:To win a war, we must first know our enemy well. Duration of Paper 1 MCQ: 1 h Duration of Paper 2: 1 h 45 min MCQ: 40 marks (30% of overall grade) Section A + B: 50 + 30 marks (50% of overall grade) A copy of the Periodic Table of Elements will be provided. What can I expect? MCQ consists of 40 questions from all topics. (Tip: Do the Mole Calculation questions LAST!) Section A consists of 7-9 compulsory structured questions, each with several parts. Section B consists of 3 questions. -The first is a compulsory data-based question and the second is a long compulsory questions. -The data-based question requires students to interpret, evaluate or solve problems using a set of given data. -The third has an either/or option. 2. Know the connection between the topicsMore challenging questions will require students to apply content knowledge from more than 1 topic. Examples: -Elements, Compounds and Mixtures + Separation Techniques -Atomic Structure + Chemical Bonding -Periodic Table + Chemical Bonding -Acids, Bases and Salts + Qualitative Analysis -Salts + Ammonia -Metals + Redox -Redox + Electrolysis -Chemical calculations + almost any topic 3. Have a copy of personalised Periodic TableBe familiar with the Periodic Table and save time by knowing where the common elements are.
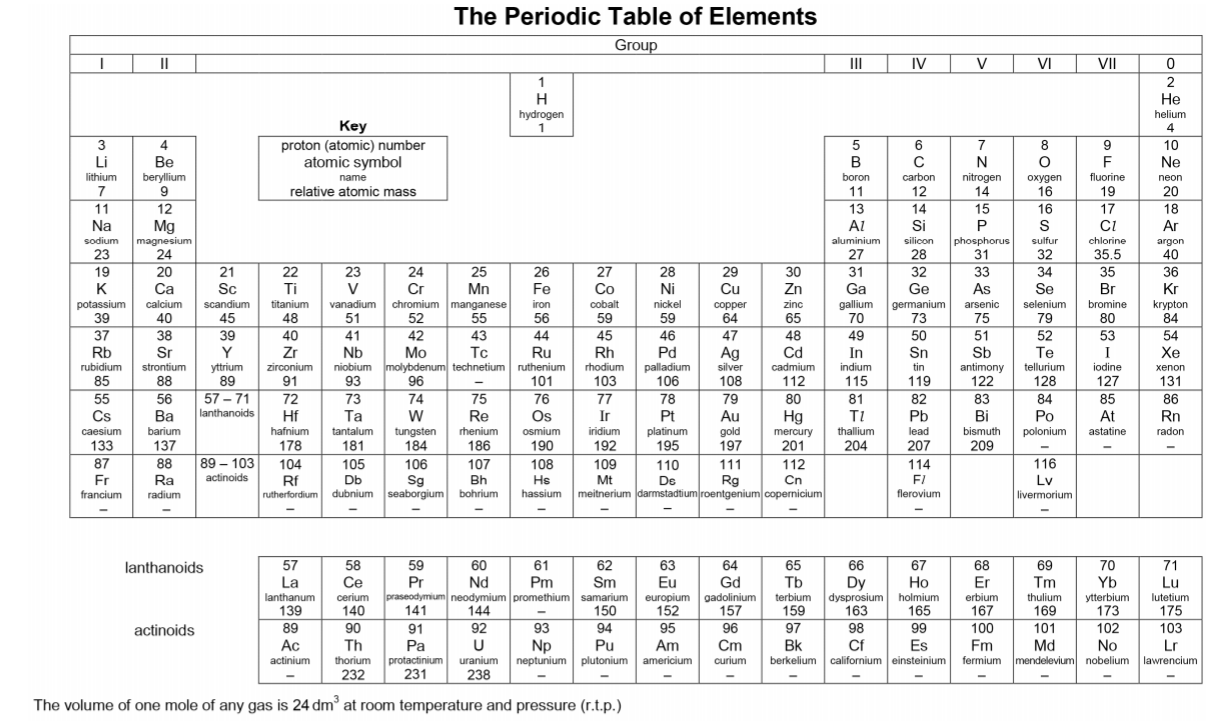
Create your own personalised Periodic Table. Jot down essential notes and refer to it for all assignments. (All my students will have a copy each from the first session.) Know exactly what information can be found on the Periodic Table: -proton/atomic number -mass/nucleon number -electronic configuration -charge of an ion -relative atomic mass -group and period number -number of electron shells and valence electrons Comments are closed.
|
Mrs Lim
A Chem-Addict passionate about teaching and learning Chemistry. Archives
February 2023
Categories |
- Home
- More Information
- Contact Me
- Blog
-
FREE 5-MIN Notes
- 1. Experimental Techniques
- 2. Methods of Purification
- 3. Separation Techniques
- 4. Qualitative Analysis
- 5. Kinetic Particle Theory
- 6. Atomic Structure
- 7. Elements, Compounds, Mixtures
- 8. Ionic Bonding
- 9. Covalent Bonding
- 10. Mole Concept
- 11. Electrolysis
- 12. Energy Changes
- 13. Speed of Reaction
- 14. Redox
- 15. Acids and Bases
- 16. Salts
- 17. Ammonia
- 18. Periodic Table
- 19. Metals
- 20. Air
- 21. Fuels
- 22. Organic Chemistry
- 23. Macromolecules
- Free Printables


 RSS Feed
RSS Feed
