Exam FormatN Level Science (Chemistry) - Sec 4 N(A)Total duration: 1 h 15 min (One paper Only)
The remaining 50% of the Science grade comes from either Physics or Biology component. Students only need to sit for 2 papers for Science (Chemistry + Physics or Biology). O Level Science (Chemistry) - Sec 5 N(A) or Sec 4 O Level TrackTotal duration for MCQ paper: 1 h
Total duration for Structured paper: 1 h 15 min
Practical Assessment - For O Level Science (Chemistry) onlyTotal duration: 1 h 30 min 30 marks - 15% of Science grade Format: 1 or 2 Questions on Chemistry (15 marks) + 1 or 2 Questions on Physics/Biology (15 marks) Implications?
Addition of New Topics1. Energy Changes 2. Speed of Reaction 3. Redox 4. Alcohols 5. Carboxylic Acids 6. Qualitative Analysis Extension of Old TopicsExperimental Design - Know how to design experiment to measure rate of reaction Identification of Ions and Gases - Describe tests for ammonia, chlorine and sulfur dioxide Mole Calculations - Formulas - Concept of limiting and excess reagent Alkenes - Addition polymerisation - Pollution problems caused by plastics These topics are exactly the same as those studied by Sec 4 O Level students. However, the questions set for these questions are no longer as simple as those seen in Sec 4 N(A) papers. Students are expected to answer questions which require them to "explain", "describe", "elaborate" or "suggest". Difficulties faced by Sec 5 students
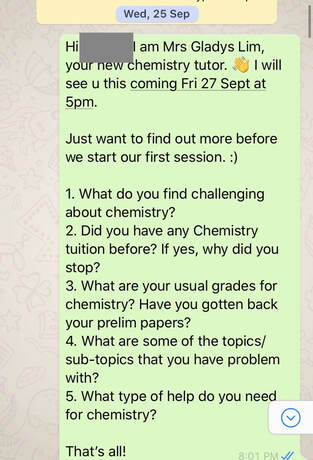
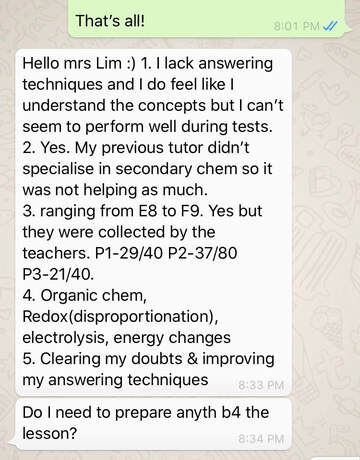
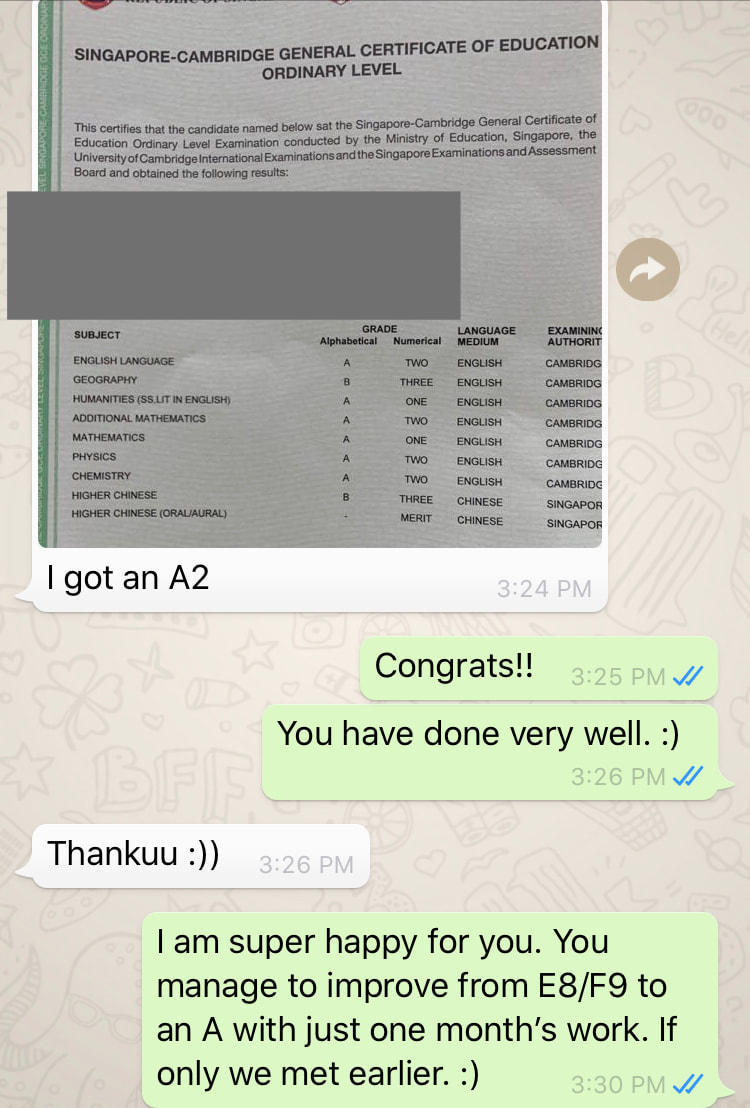
Student ProfileName: J Level: Sec 4 School: Chung Cheng High Main Grades: E8-F9 Marks for Prelims: MCQ 29/40, P2 - 37/80 (Failed), Practical 21/40(Just pass) Date of first session: 27/9/19 Total number of lessons: 8 (27/9, 4/10, 6/10, 9/10, 13/10, 16/10, 18/10 and 26/10) Total number of hours: 16 Date of O level Exam: 30/10/19 Strategy
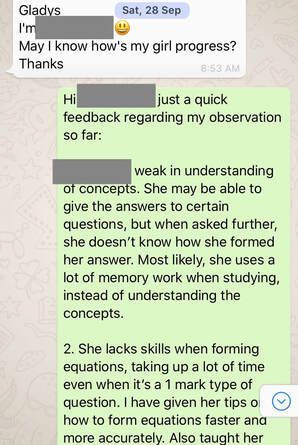
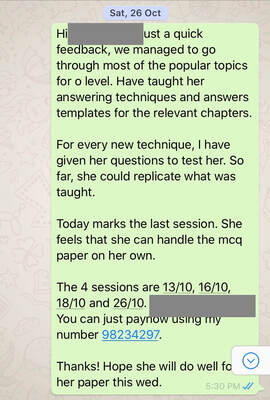
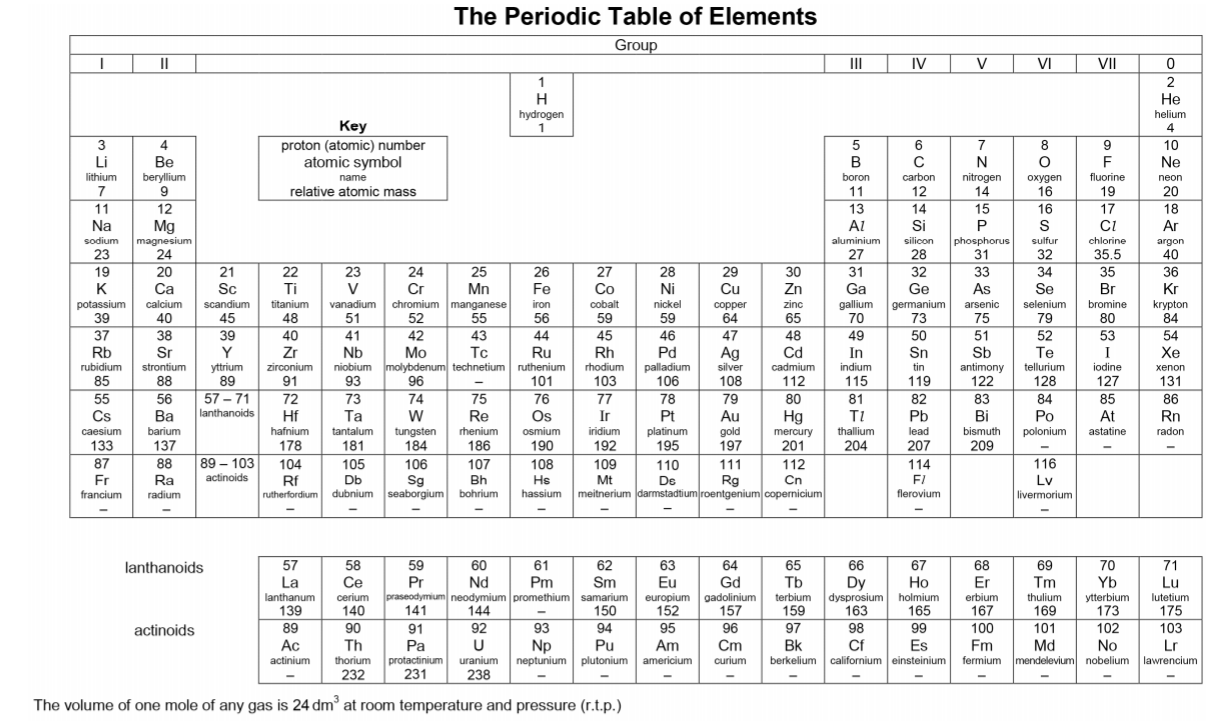
1st Feedback to Parent on 28/9/19Hi Mdm W, just a quick feedback regarding my observation so far: 1. J is weak in understanding of concepts. She may be able to give the answers to certain questions, but when asked further, she doesn’t know how she formed her answer. Most likely, she uses a lot of memory work when studying, instead of understanding the concepts. 2. She lacks skills when forming equations, taking up a lot of time even when it’s a 1 mark type of question. I have given her tips on how to form equations faster and more accurately. Also taught her how to check the equations formed. 3. She is not very sure of the keywords to use for answering questions. In chemistry, there are certain sentence structure template that we use to answer questions. And most likely she lost marks when she did not answer using important keywords (which she didn’t know that they are keywords). 4. I went through the basics to make sure that she doesn’t make the same type of mistakes again and again as these basics are repeated throughout all topics. 5. We started with the most challenging chapter which is electrolysis. She couldn’t answer well even for the most basic type of questions. Have already gone through her mistakes and given her some homework. 6. I stressed to her the importance of understanding of concepts. Knowing how to explain how she came out with the answers is crucial as the exam questions set in recent years require students to answer questions base on new context. Remembering answers from familiar context will give her a disadvantage. I gave her some reference materials, notes and worksheets. Hopefully she will find them useful. Will be preparing according to her request for the next session. 2nd Feedback on 5/10/19Hi Mdm W, just a quick update: We finished the chapter on electrolysis yesterday. It’s the hardest topic of the whole syllabus. She didn’t understand how to derive certain answers at first and by now, she should be able to as I have shared with her a method to figure out which thinking process to use. Have also given her some practice questions to do. We also covered a component of practical skill - planning. She has problem writing in a scientific way. Have gone through the requirements with her so that she knows what to write. We will move on to her next weakest topic and I will also give her more examples for practical planning question. 3rd Feedback on 10/10/19Hi Mdm W, just a quick update: I gave J a few more planning questions as example so that she has more exposure to questions. Also gave her practical exam notes and went through with her the common mistakes that students make during practical. I also tested her on the way she writes her exam answer for practical. For the theory paper, we are currently doing organic chemistry which is a large topic comprising of 6 smaller topics. I gave her homework on this topic and she’s learning from mistakes made in the homework. I also reteach a few concepts like isomers and esterification as these are her weaknesses. Yesterday was the 4th session. (27/9, 4/10, 6/10, 9/10). 4th Feedback on 15/10/19Hi Mdm W, just a quick update: 1) I gave her 2 model answers for planning exercise and both types came out for the practical exam - thermal decomposition and thermometric titration. 2) Currently we are doing the more challenging concepts for organic chemistry. Have also gone through and given her extra notes on concepts that are taken out of the syllabus but frequently tested (moe wants students to be able to analyse and do without being taught). J has come across such questions in her tests and exams before but couldn’t understand at first. Now she can do questions given without referring to notes. 3) Will be moving on to other frequently tested topics and concepts. Also gave her tips on how to study for Chemistry and what to take note of. Last Feedback on 26/10/19Hi Mdm W, just a quick feedback, we managed to go through most of the popular topics for o level. Have taught her answering techniques and answers templates for the relevant chapters. For every new technique, I have given her questions to test her. So far, she could replicate what was taught. Today marks the last session. She feels that she can handle the mcq paper on her own. The 4 sessions are 13/10, 16/10, 18/10 and 26/10. Thanks! Hope she will do well for her paper this wed. O Level Results - A Jump from E8/F9 to A2 in Just 1 MonthThe study of chemistry requires students to apply a vast amount of content knowledge to higher order thinking questions using a set of answering techniques. How can we score in Chemistry then? 1. Know the Essential Facts:To win a war, we must first know our enemy well. Duration of Paper 1 MCQ: 1 h Duration of Paper 2: 1 h 45 min MCQ: 40 marks (30% of overall grade) Section A + B: 50 + 30 marks (50% of overall grade) A copy of the Periodic Table of Elements will be provided. What can I expect? MCQ consists of 40 questions from all topics. (Tip: Do the Mole Calculation questions LAST!) Section A consists of 7-9 compulsory structured questions, each with several parts. Section B consists of 3 questions. -The first is a compulsory data-based question and the second is a long compulsory questions. -The data-based question requires students to interpret, evaluate or solve problems using a set of given data. -The third has an either/or option. 2. Know the connection between the topicsMore challenging questions will require students to apply content knowledge from more than 1 topic. Examples: -Elements, Compounds and Mixtures + Separation Techniques -Atomic Structure + Chemical Bonding -Periodic Table + Chemical Bonding -Acids, Bases and Salts + Qualitative Analysis -Salts + Ammonia -Metals + Redox -Redox + Electrolysis -Chemical calculations + almost any topic 3. Have a copy of personalised Periodic TableBe familiar with the Periodic Table and save time by knowing where the common elements are.
Create your own personalised Periodic Table. Jot down essential notes and refer to it for all assignments. (All my students will have a copy each from the first session.) Know exactly what information can be found on the Periodic Table: -proton/atomic number -mass/nucleon number -electronic configuration -charge of an ion -relative atomic mass -group and period number -number of electron shells and valence electrons |
Mrs Lim
A Chem-Addict passionate about teaching and learning Chemistry. Archives
February 2023
Categories |
- Home
- More Information
- Contact Me
- Blog
-
FREE 5-MIN Notes
- 1. Experimental Techniques
- 2. Methods of Purification
- 3. Separation Techniques
- 4. Qualitative Analysis
- 5. Kinetic Particle Theory
- 6. Atomic Structure
- 7. Elements, Compounds, Mixtures
- 8. Ionic Bonding
- 9. Covalent Bonding
- 10. Mole Concept
- 11. Electrolysis
- 12. Energy Changes
- 13. Speed of Reaction
- 14. Redox
- 15. Acids and Bases
- 16. Salts
- 17. Ammonia
- 18. Periodic Table
- 19. Metals
- 20. Air
- 21. Fuels
- 22. Organic Chemistry
- 23. Macromolecules
- Free Printables







 RSS Feed
RSS Feed
