What does Assessment of Planning mean in Practical Exam? The practical assessment incorporates assessment of Planning which has a weighting of 15% out of the entire paper. One, or more, of the questions may be set and require students to apply and integrate knowledge and understanding from different sections of the syllabus. Many students find it IMPOSSIBLE to do well in this section. And this is not true. What are the requirements for Assessment of Planning? Students are expected to be able to: • identify key variables for a given question/problem • outline an experimental procedure to investigate the question/problem • describe how the data should be used in order to reach a conclusion • identify the risks of the experiment and state precautions that should be taken to keep risks to a minimum Any example? It's quite confusing.. A typical question could be: "Plan and produce a procedure to investigate the percentage yield of copper(II) oxide using appropriate reagents and chemicals in the laboratory. You are given a sample of copper(II) carbonate, P." Wow! How do we go about doing the question? It is important to plan BEFORE writing your answers down. Just like planning for a composition. A systematic thinking process would be:
Step 1: Ask yourself this question -> What is the objective of the experiment? - To investigate the percentage yield of copper(II) oxide Step 2: THINK of the general approach that you would want to use for your experiment by applying scientific concepts that you have learnt.
Step 3: Identify variable(s) and conditions that will affect the experiment.
Step 4: Write your suggested procedure as a series of step-by-step instructions.
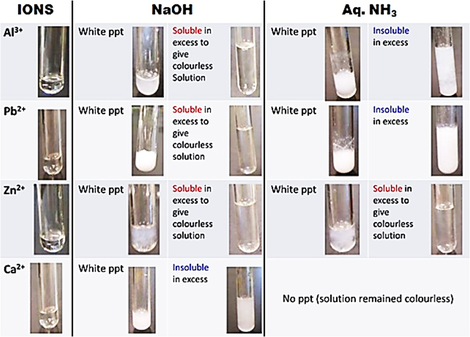
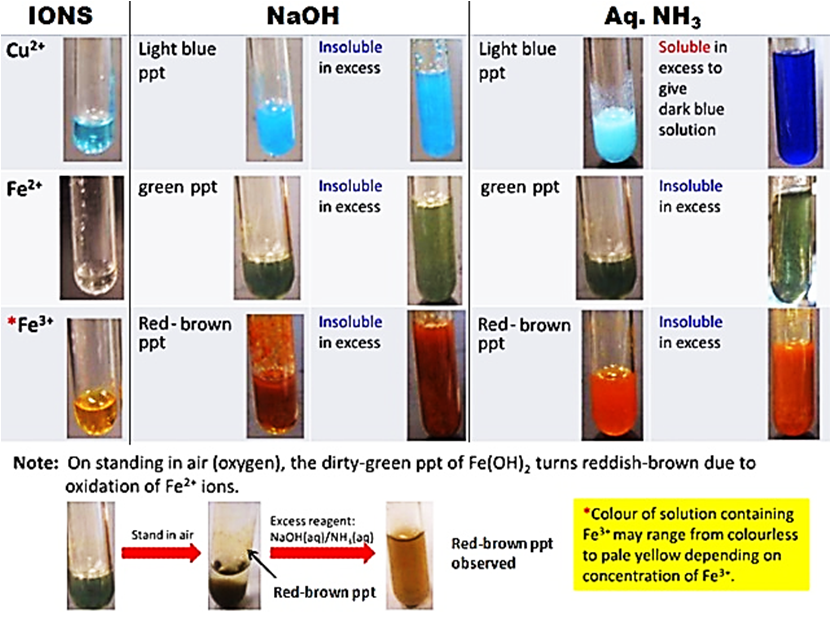
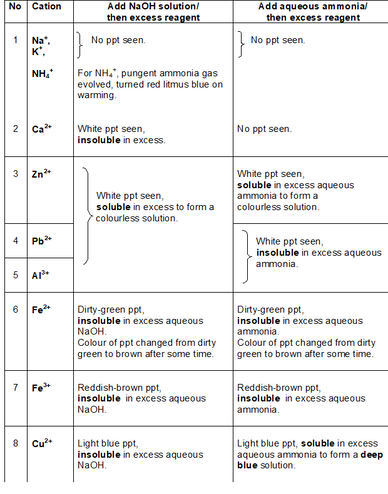
In my next post, I will be sharing more on HOW the procedure should be written. How do the precipitates look like? A picture tells a thousand words. Tip: My students will save these 2 tables in their phones for reference. What should we record for cation test observations?
How do we memorise everything? Although a simplified QA table is given during practical exam, I will always make my students understand this table thoroughly before sitting for the practical exam. If a student requires the QA table during the exam, it only means that he is not ready or confident enough. Many students memorise the table above but do not understand the concept behind. Have you ever wondered why does Ca2+ ions form a precipitate with aqueous sodium hydroxide but not with aqueous ammonia? Calcium hydroxide is slightly soluble in water and would require a high concentration of hydroxide ions to react with it to form a precipitate. Hence, since sodium hydroxide is a strong alkali, the calcium ions react with the high concentration of hydroxide ions to form a precipitate. Aqueous ammonia being a weak alkali do not have sufficient hydroxide ions and thus, no precipitate is seen. Why does the white precipitate dissolve in excess aqueous sodium hydroxide to form a colourless solution for zinc ion, aluminium ion and lead(II) ion then? The white precipitate is the hydroxide of zinc, aluminium and lead(II) which can react with aqueous sodium hydroxide to form soluble complex ions as they are amphoteric hydroxides. Do put in extra effort for Qualitative Analysis. There is a chance that it may appear both in theory and practical exam!
Many students are uncertain of what sources of error are and what to write during practical exam. This article will give your child more confidence when he has some examples of sources of error in his mind when he sits for the practical exam. What are sources of error?Sources of error are errors that are inherent in an experiment due to the DESIGN of the experiment. We need to suggest IMPROVEMENT to reduce the sources of error in the experiment. What are NOT sources of error? Sources of error are not due to the mistakes made by the student conducting the experiment. Today, I am going to share with you 3 aspects of design which can cause sources of error to occur. 1. Apparatus There are times when an apparatus is chosen due to the ease of use. Hence, the degree of accuracy may be compromised and this can be a source of error for the experiment. Example: When a measuring cylinder is used to measure 25 cm^3 of a solution instead of a pipette, we can state the source of error this way -> The measuring cylinder used to measure the volume of solution is not as precise as a pipette. It can only measure accurately to 1 cm^3. The improvement will be -> Use a pipette instead of a measuring cylinder to measure the 25.0 cm^3 volume. 2. Reagents The stability of the reagent used can be a possible source of error if
Example: Hydrogen peroxide decomposes slowly into water and oxygen at room temperature even when no catalyst is added. The rate of decomposition increases with light and heat. Hence, the source of error will be -> Since hydrogen peroxide decomposes into water and oxygen at room temperature, there will be a loss of reagent before and during the experiment. The improvement will be -> Store hydrogen peroxide in a dark glass bottle, away from light and heat. 3. Dependent variables When the dependent variable is temperature change, the source of error can be caused by the inherent inaccuracy when temperature is measured.
Example: Heat is always being lost to the surrounding when the surrounding is cooler compared to the reacting mixture. The source of error can be written as -> Heat lost to the surrounding is caused by the poor insulation of the reactants, hence the temperature measured will be lower than expected. The suggested improvement will be -> Use a polystyrene cup with a cover to minimise heat lost to the surrounding. |
Mrs Lim
A Chem-Addict passionate about teaching and learning Chemistry. Archives
February 2023
Categories |
- Home
- More Information
- Contact Me
- Blog
-
FREE 5-MIN Notes
- 1. Experimental Techniques
- 2. Methods of Purification
- 3. Separation Techniques
- 4. Qualitative Analysis
- 5. Kinetic Particle Theory
- 6. Atomic Structure
- 7. Elements, Compounds, Mixtures
- 8. Ionic Bonding
- 9. Covalent Bonding
- 10. Mole Concept
- 11. Electrolysis
- 12. Energy Changes
- 13. Speed of Reaction
- 14. Redox
- 15. Acids and Bases
- 16. Salts
- 17. Ammonia
- 18. Periodic Table
- 19. Metals
- 20. Air
- 21. Fuels
- 22. Organic Chemistry
- 23. Macromolecules
- Free Printables



 RSS Feed
RSS Feed
