|
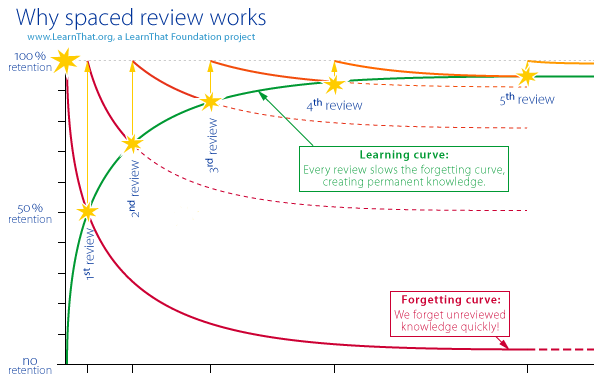
School holiday is here. It means that the chance of doing a proper revision has finally arrived! So, how should your child start revising for Chemistry? Step 1: "Curate" a strong set of notes I read an article on the latest trend about #studygram and was amazed. Is this the trendy way to study nowadays? Article link -> www.straitstimes.com/lifestyle/welcome-to-my-studygram In my opinion, writing and decorating such beautiful notes as a study method is not sustainable at all. The focus is just so wrong. So much effort is used to decorate the notes and not "curating" the information that makes a difference to your results. For my students, I always encourage them to use notes provided by their schools (if they pass my Quality Check) so that they will use the exact keywords expected from their teachers. You'll be surprised that different school teachers have different sets of keywords. For some schools, the teachers provide notes which are either too brief or too elaborated. In worst cases, the teacher just asked the students to refer to their textbooks! (Yes, and that teacher is currently still a HOD of Science in the East.) My job is to help my students "curate" a strong set of notes. I will provide my notes which will complement the notes provided by the school. All my notes are customised for my students depending on their needs. Together, we will analyse notes provided by the school and add in information (from my notes, ten-year series, exam questions, school worksheets) which are crucial for getting distinction. At the same time, I will also highlight the parts which are not important at all to minimise effort for studying. Why do you need a good set of notes? Strong notes are the core of a good study regime. A good set of notes does not mean just a set of stapled notes per topic. It includes notes written on A4 exercise books too. All these should be "curated" from the start of the year, starting from the first topic. What type of materials does your child use for revision? Start "curating" now before it's too late! Step 2: Do practice questions frequently (Make it a routine) I'm a firm believer of the Spaced Review Concept. It's impossible to retain information if we do not review them. Many students may not believe this at first, thinking that it's just common sense. This is actually proven scientifically. From the Forgetting Curve Theory, forgetting happens most rapidly right after learning occurs and slows as time passes. The more we review it, the longer we can remember it. Where can I find practice questions for Chemistry revision?
Please, please, please DO NOT trust the answers from Ten-Year Series Publishers. There are lots and lots of mistakes especially for the structured questions. Not minor ones, but those that will cause misconceptions. The way the answers are structured is very different from what we expect in school. There's no way a student can learn how to answer properly by looking at the answers provided. Many students and parents think that the answers provided are from the examination board. NO! That's not true. The examination board only provides the questions and NOT the answers. I discourage students from wasting their money buying assessment books. Many assessment books are published by random authors who are not NIE-trained. The questions are very different from what teachers set during exams. Do not study using notes from assessment books. You will never be sure where the source is from. For my students, my first priority is for them to do my worksheets and test papers, followed by the ten-year series. I will mark the ten-year series using the guidelines from the marker's report and provide immediate feedback for your child. The more feedback you get the more you’ll improve! Is your child's ten-year series still in pristine condition? If your child dedicates at least 2 hours a week to do the above 2 steps during my lesson, he will retain much more due to constant exposure to my quality notes, practice questions and answers. Contact me if you're looking for extra help with your child's revision. General Tips
Common Mistakes for MCQ
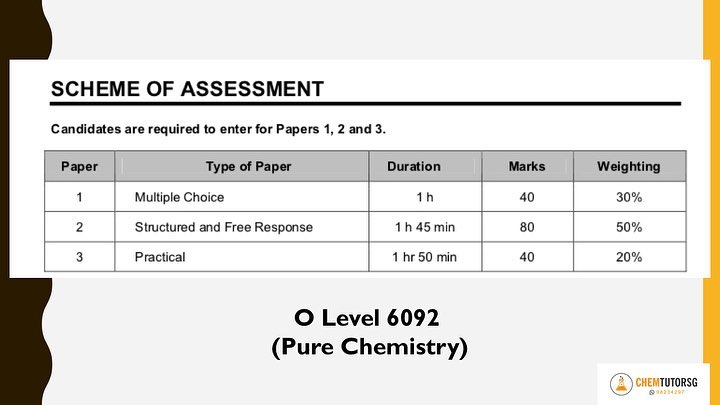
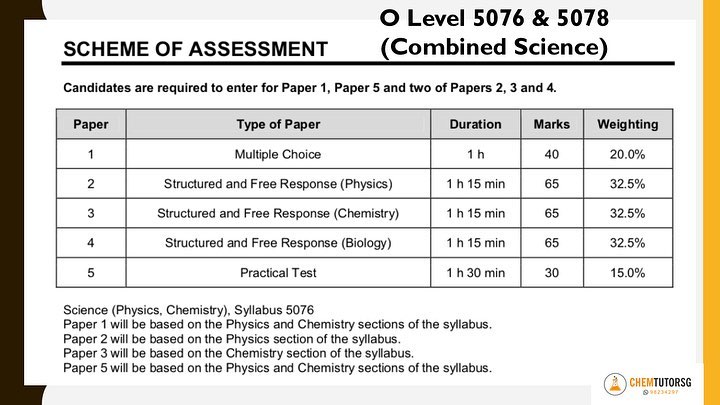
Hope you find these useful! Will share more tips for structured questions in the future. Recently, I got a call from a desperate mother who found my number through this website. Her daughter has not been doing well for Pure Chemistry and the school has advised her to drop to combined Science (chemistry/biology). "What should I advise my child?" she asked me. In order to give a good advice, there are multiple factors to consider. 1. How different are the syllabuses? The syllabus content of combined Chemistry is approximately 75% of the whole Pure Chemistry syllabus. Obviously, your child will need to study less content. What exactly is the difference? The whole topic on the most hated ELECTROLYSIS will be removed! Yay! Other significant portions removed are:
2. How different are the exam requirements? Differences based on the scheme of assessment:
Scheme of Assessment taken from SEAB Website 3. Will dropping to combine science affect entry to JC/Poly? Yes, it will. By dropping to combine Science, the subject combination for JC entry will be more limited. (Do check the entry and subject combination requirements from the respective official school websites.) However, some students are very sure of the path that they are going to take once they complete their secondary school education. Thus, they may choose to pursue their interest in theater studies, humanities etc. So, it's important to discuss your child’s strengths as a family and decide base on your child's ambitions and interests and not choose base on what others will do! 4. My thoughts? Combined Science is so much easier to score. It's quite common for students who dropped to combine Science to score better as these students have more in depth knowledge compared to their peers who have only studied the combine Chemistry syllabus before. Many tutors may think that by dropping from two subjects to one subject, your child will be taking a huge risk as there's only one science subject for the L1R5 calculation. But in my opinion, it's much easier to score an A1 or A2 for combine Chemistry compared to scoring an A1 or A2 or even B3 for pure Chemistry. I'm confident to say that as all my combine Chemistry students score 100% distinctions despite coming from different backgrounds. Some met me after dropping from Pure Chemistry and others have taken combine Chemistry from the start. 5. When should I DISCOURAGE my child to drop to combine Science? There are some students who are doing poorly for both Sciences. In this case, it is wisest to drop to combine Science for sure. However, if your child is doing very well and scoring distinctions for one of the Pure Sciences, I will suggest not to waste that effort already put in and continue to take up 2 Pure Sciences. Encourage your child to put in more time and effort for the weaker Science using whatever that worked for the stronger Science. It is essential to find a good tutor in order for your child to clarify doubts once and for all. If your child can be good at one Science, he will most likely have the skills and aptitude to score well in the other. Sometimes, it also depends on the difficulty of the papers set. In certain schools, the papers are set to 'kill' while in others, the papers are so much easier compared to the O level standard. In this case, you will need an experience tutor to analyse the paper to know the 'actual' standard of your child. Do not base just on the percentage written on the paper itself. It may or may not be an accurate representation. 6. What did you advise that particular student? During my first session with the student, I looked through her Secondary 3 to 4 tests and exam papers. I also analysed her Secondary 4 Pure Chemistry mid-year papers which she just received back. I advised her to drop to combine Science. Why?
She is currently having intensive lessons with me and we are making fast progress. Our aim? A1 for combine Science. Nothing less than that. I hope I have given you a balanced viewpoint based on my own experiences and opinions. In short, there's no right or wrong way to decide whether to drop to combine Science. It all depends...
Contact me if your child needs someone for advice. What does Assessment of Planning mean in Practical Exam? The practical assessment incorporates assessment of Planning which has a weighting of 15% out of the entire paper. One, or more, of the questions may be set and require students to apply and integrate knowledge and understanding from different sections of the syllabus. Many students find it IMPOSSIBLE to do well in this section. And this is not true. What are the requirements for Assessment of Planning? Students are expected to be able to: • identify key variables for a given question/problem • outline an experimental procedure to investigate the question/problem • describe how the data should be used in order to reach a conclusion • identify the risks of the experiment and state precautions that should be taken to keep risks to a minimum Any example? It's quite confusing.. A typical question could be: "Plan and produce a procedure to investigate the percentage yield of copper(II) oxide using appropriate reagents and chemicals in the laboratory. You are given a sample of copper(II) carbonate, P." Wow! How do we go about doing the question? It is important to plan BEFORE writing your answers down. Just like planning for a composition. A systematic thinking process would be:
Step 1: Ask yourself this question -> What is the objective of the experiment? - To investigate the percentage yield of copper(II) oxide Step 2: THINK of the general approach that you would want to use for your experiment by applying scientific concepts that you have learnt.
Step 3: Identify variable(s) and conditions that will affect the experiment.
Step 4: Write your suggested procedure as a series of step-by-step instructions.
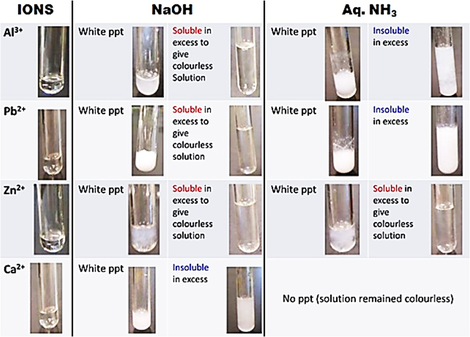
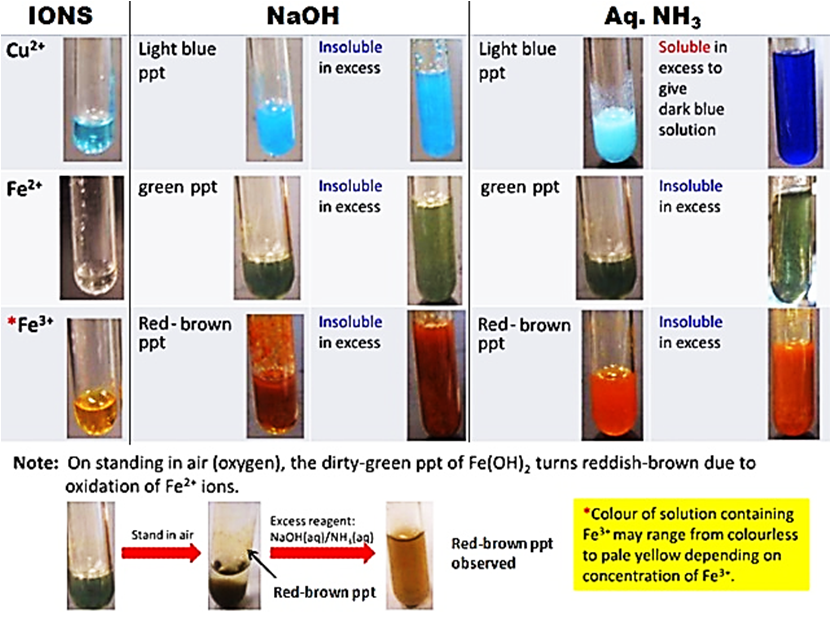
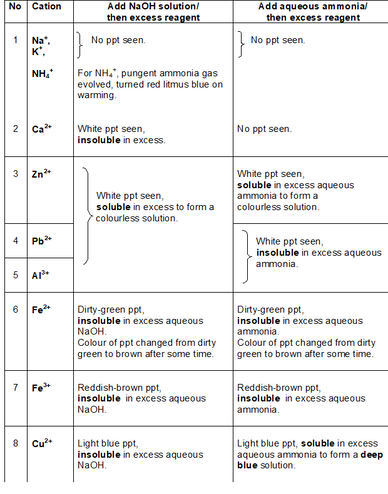
In my next post, I will be sharing more on HOW the procedure should be written. How do the precipitates look like? A picture tells a thousand words. Tip: My students will save these 2 tables in their phones for reference. What should we record for cation test observations?
How do we memorise everything? Although a simplified QA table is given during practical exam, I will always make my students understand this table thoroughly before sitting for the practical exam. If a student requires the QA table during the exam, it only means that he is not ready or confident enough. Many students memorise the table above but do not understand the concept behind. Have you ever wondered why does Ca2+ ions form a precipitate with aqueous sodium hydroxide but not with aqueous ammonia? Calcium hydroxide is slightly soluble in water and would require a high concentration of hydroxide ions to react with it to form a precipitate. Hence, since sodium hydroxide is a strong alkali, the calcium ions react with the high concentration of hydroxide ions to form a precipitate. Aqueous ammonia being a weak alkali do not have sufficient hydroxide ions and thus, no precipitate is seen. Why does the white precipitate dissolve in excess aqueous sodium hydroxide to form a colourless solution for zinc ion, aluminium ion and lead(II) ion then? The white precipitate is the hydroxide of zinc, aluminium and lead(II) which can react with aqueous sodium hydroxide to form soluble complex ions as they are amphoteric hydroxides. Do put in extra effort for Qualitative Analysis. There is a chance that it may appear both in theory and practical exam!
Many students are uncertain of what sources of error are and what to write during practical exam. This article will give your child more confidence when he has some examples of sources of error in his mind when he sits for the practical exam. What are sources of error?Sources of error are errors that are inherent in an experiment due to the DESIGN of the experiment. We need to suggest IMPROVEMENT to reduce the sources of error in the experiment. What are NOT sources of error? Sources of error are not due to the mistakes made by the student conducting the experiment. Today, I am going to share with you 3 aspects of design which can cause sources of error to occur. 1. Apparatus There are times when an apparatus is chosen due to the ease of use. Hence, the degree of accuracy may be compromised and this can be a source of error for the experiment. Example: When a measuring cylinder is used to measure 25 cm^3 of a solution instead of a pipette, we can state the source of error this way -> The measuring cylinder used to measure the volume of solution is not as precise as a pipette. It can only measure accurately to 1 cm^3. The improvement will be -> Use a pipette instead of a measuring cylinder to measure the 25.0 cm^3 volume. 2. Reagents The stability of the reagent used can be a possible source of error if
Example: Hydrogen peroxide decomposes slowly into water and oxygen at room temperature even when no catalyst is added. The rate of decomposition increases with light and heat. Hence, the source of error will be -> Since hydrogen peroxide decomposes into water and oxygen at room temperature, there will be a loss of reagent before and during the experiment. The improvement will be -> Store hydrogen peroxide in a dark glass bottle, away from light and heat. 3. Dependent variables When the dependent variable is temperature change, the source of error can be caused by the inherent inaccuracy when temperature is measured.
Example: Heat is always being lost to the surrounding when the surrounding is cooler compared to the reacting mixture. The source of error can be written as -> Heat lost to the surrounding is caused by the poor insulation of the reactants, hence the temperature measured will be lower than expected. The suggested improvement will be -> Use a polystyrene cup with a cover to minimise heat lost to the surrounding. Does your child know ALL of these crucial tips?These are some general tips which I will go through with my students during lessons using concrete examples. Share these tips with your child so that he is AWARE of the REQUIREMENTS of the examination.
|
Mrs Lim
A Chem-Addict passionate about teaching and learning Chemistry. Archives
February 2023
Categories |
- Home
- More Information
- Contact Me
- Blog
-
FREE 5-MIN Notes
- 1. Experimental Techniques
- 2. Methods of Purification
- 3. Separation Techniques
- 4. Qualitative Analysis
- 5. Kinetic Particle Theory
- 6. Atomic Structure
- 7. Elements, Compounds, Mixtures
- 8. Ionic Bonding
- 9. Covalent Bonding
- 10. Mole Concept
- 11. Electrolysis
- 12. Energy Changes
- 13. Speed of Reaction
- 14. Redox
- 15. Acids and Bases
- 16. Salts
- 17. Ammonia
- 18. Periodic Table
- 19. Metals
- 20. Air
- 21. Fuels
- 22. Organic Chemistry
- 23. Macromolecules
- Free Printables









 RSS Feed
RSS Feed
