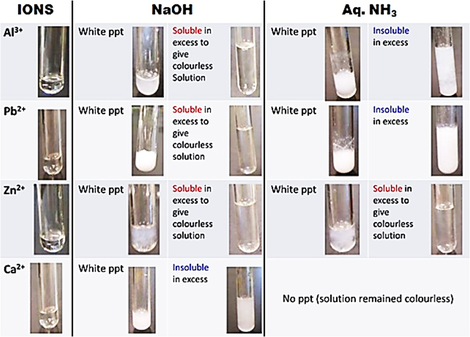
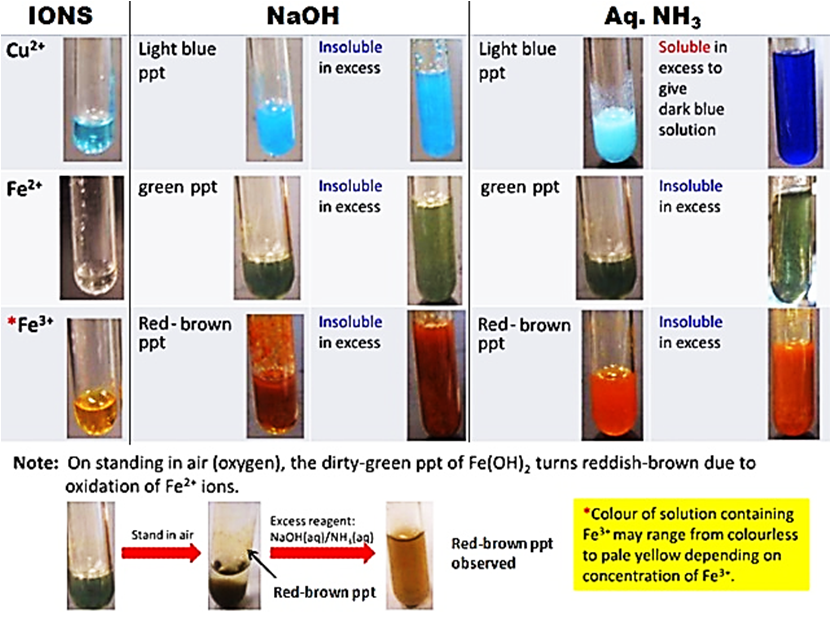
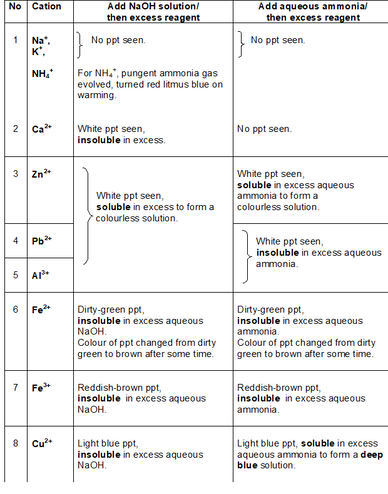
How do the precipitates look like? A picture tells a thousand words. Tip: My students will save these 2 tables in their phones for reference. What should we record for cation test observations?
How do we memorise everything? Although a simplified QA table is given during practical exam, I will always make my students understand this table thoroughly before sitting for the practical exam. If a student requires the QA table during the exam, it only means that he is not ready or confident enough. Many students memorise the table above but do not understand the concept behind. Have you ever wondered why does Ca2+ ions form a precipitate with aqueous sodium hydroxide but not with aqueous ammonia? Calcium hydroxide is slightly soluble in water and would require a high concentration of hydroxide ions to react with it to form a precipitate. Hence, since sodium hydroxide is a strong alkali, the calcium ions react with the high concentration of hydroxide ions to form a precipitate. Aqueous ammonia being a weak alkali do not have sufficient hydroxide ions and thus, no precipitate is seen. Why does the white precipitate dissolve in excess aqueous sodium hydroxide to form a colourless solution for zinc ion, aluminium ion and lead(II) ion then? The white precipitate is the hydroxide of zinc, aluminium and lead(II) which can react with aqueous sodium hydroxide to form soluble complex ions as they are amphoteric hydroxides. Do put in extra effort for Qualitative Analysis. There is a chance that it may appear both in theory and practical exam!
Comments are closed.
|
Mrs Lim
A Chem-Addict passionate about teaching and learning Chemistry. Archives
February 2023
Categories |
- Home
- More Information
- Contact Me
- Blog
-
FREE 5-MIN Notes
- 1. Experimental Techniques
- 2. Methods of Purification
- 3. Separation Techniques
- 4. Qualitative Analysis
- 5. Kinetic Particle Theory
- 6. Atomic Structure
- 7. Elements, Compounds, Mixtures
- 8. Ionic Bonding
- 9. Covalent Bonding
- 10. Mole Concept
- 11. Electrolysis
- 12. Energy Changes
- 13. Speed of Reaction
- 14. Redox
- 15. Acids and Bases
- 16. Salts
- 17. Ammonia
- 18. Periodic Table
- 19. Metals
- 20. Air
- 21. Fuels
- 22. Organic Chemistry
- 23. Macromolecules
- Free Printables

 RSS Feed
RSS Feed
